Abstract
Introduction:
DOACs are absorbed in the gastrointestinal (GI) tract and DOAC elimination is primarily through the GI and genitourinary (GU) systems. The safety of DOACs in cancer associated thrombosis in subjects with malignant lesions in GI and GU malignancies has been of concern. Studies have been sparse and data conflicting.
Methods:
We identified patients with active GI and GU malignancies from July 2001 to July 2020 with confirmed VTE at our institution. Patients who received either enoxaparin or a DOAC (apixaban or rivaroxaban) were included in the study. Demographic, disease characteristics, VTE data and events were extracted from electronic medical records (EMR). Date of anticoagulation (AC) initiation was based on the first order and/or prescription of the anticoagulant. Patients were followed either to the earliest bleeding event (BE) or one year from initiation, whichever occurred first. BEs were categorized based on ISTH guidelines. Variables were compared between LMWH and DOAC cohorts, as well as between the apixaban and rivaroxaban cohorts, using t-tests for continuous variables and chi-squared tests or Fisher's exact test for categorical variables.
Results:
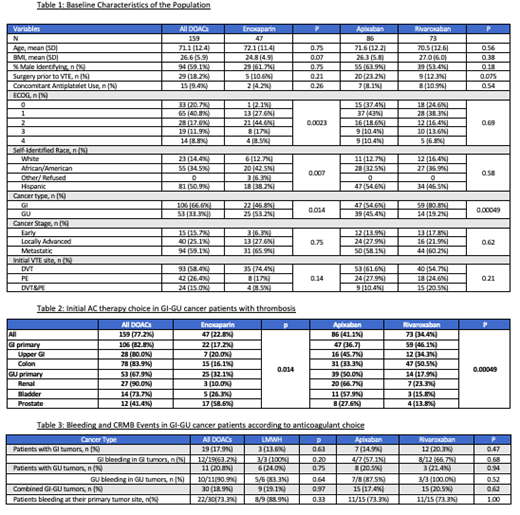
We identified a total of 206 patients, 159 in the DOAC and 47 in the LWMH groups. Table 1 describes the baseline characteristics of the study populations. Median age of patients, gender and BMI were comparable for all groups. When evaluated for type of cancer, 66.6% of patients had active GI malignancy while 33.3% had active GU tumors. The majority of the patients given DOACs had a better ECOG status than those in the LMWH group (p=0.0023), but no difference was noted for ECOG status between DOACs (p=0.69). Most patients had metastatic disease. The majority of the VTE events were in the form of DVTs. Concomitant aspirin intake was 9.4% in DOAC and 4.2% in LMWH groups. Cancer subtypes and AC choice data are given in detail in Table 2.
LMWH use was higher in blacks and somewhat lower in the Hispanic population (Table 1). When anticoagulation choice was examined by primary tumor site (Table 2), disproportionately more patients with GU tumors were placed on LMWH while more GI cancers were given a DOAC (p=0.014). Extent and stage of the cancer did not appear to bias anticoagulant choice (p=0.62). Within the DOACs, rivaroxaban use was higher in the GI cancers but considerably less used in the GU malignancies (p=0.00049).
There was one recurrent thrombosis in each of the apixaban (1/86) and the rivaroxaban (1/73) cohorts. There were no recurrent events in the LMWH (0/47) cohort.
The majority of patients in the DOAC and LMWH groups, 88.1% and 86.4%, had no CRNMB or major bleeding events in the 1-year period after the initiation of the therapeutic AC (Table 3). Combined BE (clinically relevant non-major bleeding [CRNMB] and major bleeding rates) with apixaban, rivaroxaban and LMWH were 17.4% (15/86), 20.5% (15/73), and 19.1% (9/47) respectively. There were no fatal bleeding episodes in any of the groups. Most of the bleeding events on DOACs and LMWHs occurred in the same organ system as the primary cancer (Table 3) but there was no statistically significant difference in bleeding events between patients on DOACs or LMWH for GI, GU or all cancer types (p=0.63, 0.75 and 0.97 respectively). Within DOACs, we also noted no statistically significant difference in the bleeding events with apixaban as compared to rivaroxaban in patients with GI primary, GU primary or all cancer types together, (p=0.47, 0.94 and 0.62 respectively).
Conclusion:
No significant differences in major/CRNM bleeding events were found for patients with GI or GU cancer associated thrombosis given DOACs (apixaban/rivaroxaban) vs enoxaparin. The tumor site is often the site of bleeding, but no differences in tumor-specific site bleeding could be shown by anticoagulant choice.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal